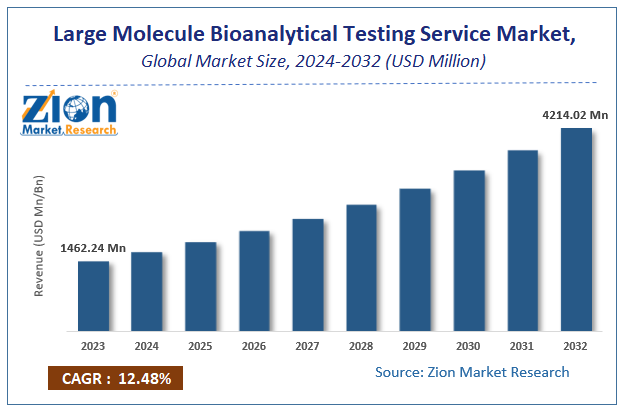
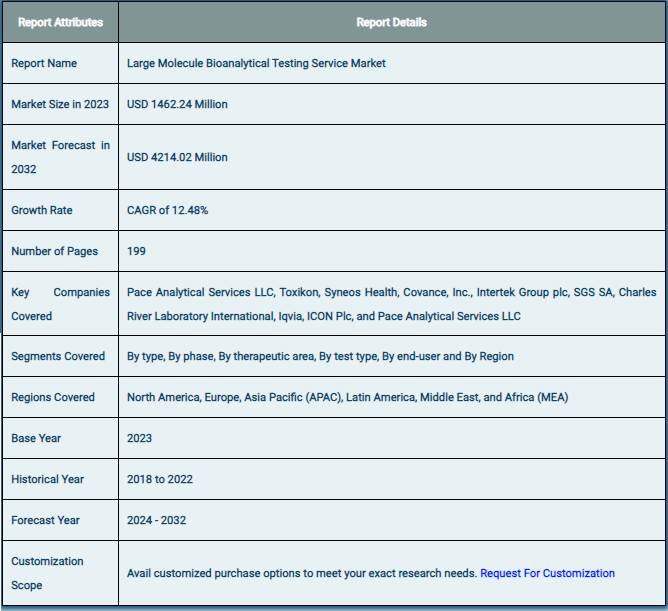
The global market for large-molecule bioanalytical testing services was estimated to be worth USD 1462.24 million in 2024 and is expected to grow to USD 4214.02 million by the end of 2032, according to a report released by Zion Market Research. Over the course of the projection period, the market is anticipated to expand at a CAGR of 12.48%. The growth factors, barriers to entry, and effects on demand for the global large molecule bioanalytical testing services market are examined in this report’s analysis. Additionally, it will support you as you navigate and investigate the new opportunities in the market for large-molecule bioanalytical testing services.
Market Overview
One area of analytical chemistry that examines large molecule medicines in a biological matrix is bioanalysis of large molecules. It alludes to a collection of protocols, techniques, and tests that enable researchers to examine the big molecules and biochemical processes that underpin biological processes. The majority of typical bioanalytical study types consist of immunogenicity testing, biomarker assay, pharmacodynamic investigations, and pharmacokinetic studies. Since large molecule products are generated on a large scale, bioanalysis is required. Clinical research organisations provide large molecule bioanalytical testing services in order to meet this need.
Large molecule bioanalytical testing services involve the analysis and testing of biological macromolecules, such as proteins, peptides, antibodies, and nucleic acids. These services are essential in drug development, particularly in the development and validation of biopharmaceuticals and biosimilars. The demand for large molecule bioanalytical testing is growing due to the increasing prevalence of biologics in the pharmaceutical pipeline and the need for specialized testing services to support drug discovery, preclinical and clinical development, and regulatory submissions.
Growth Factors for the Global Large Molecule Bioanalytical Testing Services Market
Some of the key factors driving the growth of the global large molecule bioanalytical testing service market are the rise in the use of large molecules in biopharmaceutical companies, the rise in biotech & pharmaceutical companies’ adoption of outsourcing services, and the rise in the demand for novel drugs to combat diseases. Large molecule therapeutic products such as proteins, peptides, antibody-drug conjugates, and monoclonal antibodies have shown great efficacy against a variety of disease indications. As a result, the creation of big molecular products is increasing. Furthermore, a plethora of service providers provides pharmaceutical businesses with large molecule bioanalytical testing services. The cutting-edge bioanalytical laboratories are equipped with a variety of cutting-edge platforms that maximise repeatability and accuracy by utilising several assays, such as cell-based assays, pharmacokinetics, immunogenicity, and biomarkers.
With established scientific and regulatory knowledge, the service providers confidently and swiftly assist the advancement of big molecule breakthroughs along the drug development pipeline. By providing comprehensive bioanalytical laboratory services from phase I through IV of discovery, they shorten the time it takes from discovery to market. The market is expanding as a result of all these factors. The global market is expanding due to the increasing demand for studies on immunogenicity, pharmacokinetics, toxicokinetic, and biomarker discovery. Moreover, throughout the course of the forecast period, there will be multiple chances for the expansion of the global large molecule bioanalytical testing services market due to technological improvements in the medical sciences and expanding research on the creation of novel pharmaceuticals. On the other hand, insufficient knowledge will impede the expansion of the worldwide market for large molecule bioanalytical testing services.
The Covid-19 epidemic had an impact on the growth of the big molecule bioanalytical testing services market globally in the first half of 2020. This is because governments all around the world decided to implement total lockdown and travel restrictions in an effort to stop the disease from spreading. The pharmaceutical industry’s manufacturing facilities were impacted by the interruption in the supply chain and the labour shortage. Clinical investigations and drug development initiatives were momentarily put on hold. Nonetheless, the service providers are facilitating Covid-19 clinical studies with minimal constraints, which is driving the expansion of the worldwide market.
Market Segmentation for Large Molecule Bioanalytical Testing Services Worldwide
Based on type, phase, therapeutic area, test type, end-user, and region, the global large molecule bioanalytical testing services market is divided into different segments. The worldwide market for large molecule bioanalytical testing services is divided into ADA, pharmacokinetics, and other segments based on the kind. The worldwide market is divided into four phases: preclinical, clinical, without antibody, and with antibody. The therapeutic area component is further subdivided into infectious illnesses, neurology, cardiology, and oncology. The market is divided into PD, PK, ADME, bioequivalence, bioavailability, and others depending on the type of test. There is a divide between SMEs and large companies in the end-user segment.
Market for Large-Molecule Bioanalytical Testing Services: Report Extent
Regional Analysis of the Global Market for Large Molecule Bioanalytical Testing Services
Over the course of the forecast period, North America is anticipated to dominate the global market for large molecule bioanalytical testing services. Factors include the abundance of CROs providing large molecule bioanalytical testing services, the presence of well-equipped bioanalytical laboratories, and the quick adoption of cutting-edge technology. Due to significant investments in the healthcare infrastructure and an increased emphasis on drug discovery and development initiatives, the Asia Pacific area is predicted to grow significantly. Europe is anticipated to hold a substantial portion of the market as a result of the growing trend of pharmaceutical companies outsourcing services.
Market Drivers
Several factors are contributing to the growth of the large molecule bioanalytical testing services market:
- Rising Demand for Biologics and Biosimilars: The pharmaceutical industry’s shift toward biologics and biosimilars has significantly increased the need for specialized bioanalytical testing services to ensure the safety, efficacy, and quality of these products.
- Advancements in Analytical Techniques: Technological advancements in bioanalytical methods, such as mass spectrometry, chromatography, and ligand-binding assays, have improved the sensitivity, accuracy, and throughput of large molecule testing.
- Increasing Outsourcing to Contract Research Organizations (CROs): Pharmaceutical and biotechnology companies are increasingly outsourcing their bioanalytical testing needs to CROs to reduce costs, gain access to specialized expertise, and focus on core competencies.
- Stringent Regulatory Requirements: Regulatory agencies such as the FDA and EMA have stringent guidelines for bioanalytical testing, driving the demand for high-quality testing services to ensure compliance and successful drug approvals.
Market Segmentation
The market for large molecule bioanalytical testing services can be segmented based on several criteria:
- By Service Type: Method development, validation, sample testing, pharmacokinetics/pharmacodynamics (PK/PD) testing, immunogenicity testing, biomarker testing, and stability testing.
- By Therapeutic Area: Oncology, neurology, cardiology, infectious diseases, endocrinology, and others.
- By End-User: Pharmaceutical and biotechnology companies, CROs, academic and research institutes, and others.
- By Region: North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa.
Competitive Landscape
The large molecule bioanalytical testing services market is highly competitive, with numerous players offering a wide range of services to support biologics development. Key players in the market include:
- Charles River Laboratories
- Labcorp Drug Development
- Eurofins Scientific
- SGS SA
- PPD, Inc. (Thermo Fisher Scientific)
- WuXi AppTec
- ICON plc
- Medpace Holdings, Inc.
- Intertek Group plc
These companies are focused on expanding their service offerings, enhancing their technological capabilities, and forming strategic partnerships to strengthen their market positions.
Challenges
Despite the growth prospects, the large molecule bioanalytical testing services market faces several challenges:
- High Costs and Complexity: The development and validation of bioanalytical methods for large molecules can be more complex and costly than for small molecules due to their larger size, structural complexity, and diverse range of modifications.
- Regulatory Challenges: Keeping up with evolving regulatory guidelines and ensuring compliance can be challenging for service providers, particularly in a global market with differing regional requirements.
- Data Integrity and Quality Control: Ensuring the accuracy and reliability of bioanalytical data is critical, and maintaining high standards of data integrity and quality control is a significant challenge in a competitive market.
Future Trends
The future of the large molecule bioanalytical testing services market is shaped by several emerging trends:
- Growth in Biopharmaceutical R&D: As the development pipeline for biologics continues to expand, the demand for bioanalytical testing services will likely increase, particularly for innovative therapies such as gene therapies, cell therapies, and monoclonal antibodies.
- Advances in Bioanalytical Technologies: Continued innovation in bioanalytical technologies, such as next-generation sequencing (NGS), high-resolution mass spectrometry, and novel ligand-binding assays, will drive improvements in sensitivity, specificity, and throughput, supporting more efficient drug development processes.
- Increased Focus on Biomarkers and Companion Diagnostics: The rising importance of personalized medicine and targeted therapies is driving demand for biomarker testing and companion diagnostics, which require specialized bioanalytical testing services.
Conclusion
The Large Molecule Bioanalytical Testing Services Market is poised for significant growth due to the increasing prevalence of biologics, technological advancements in bioanalytical methods, and rising demand for outsourcing to specialized service providers. However, the market faces challenges such as high costs, regulatory complexities, and the need for robust data management and quality control practices. Future growth will be driven by continued advancements in technology, expansion of biopharmaceutical R&D activities, and an increasing focus on personalized medicine.
Contact Us:
Zion Market Research212
USA/Canada Toll Free: 1 (855) 465–4651
Newark: 1 (302) 444–016611\s
Web: https://www.zionmarketresearch.com/
Blog: https://zmrblog.com/


